|
We are getting asked if it is appropriate to bill for generic/compounded aminolevulinic acid HCl prepared by a compounding pharmacy using J-code J7308, typically associated with Levulan (manufactured by Sun Pharmaceuticals).
The short answer is... no, you cannot bill using J7308!
If a dermatology office were to purchase a compounded form of ALA (aminolevulinic acid) HCl from a compounding pharmacy, there are some challenges for billing using the standardized CPT/HCPCS codes. Reimbursement is also uncertain and there is not cost advantage or 'profit' to me made by the dermatologist.
Let me explain.
There are HCPCS J-codes for two forms of ALA-type medications approved by the FDA.
J7308 - Aminolevulinic acid hcl for topical administration, 20%, single unit dosage form (354 mg) - (Typically associated with Levulan)
J7345 - Aminolevulinic acid hcl for topical administration, 10% gel, 10 mg - (new code for Ameluz)
With each of these two J-codes, there are certain National Drug Codes (NDC) that are assigned to each drug and pharmaceutical manufacturer indicating the packaging, formulation, dosage, concentration, units/package of each approved product, etc.
For example, the FDA has assigned several NDC codes for Ameluz. 70621-101-01, 70621-101-10, 70621-101-30
For Levulan, the FDA has assigned assigned a different sent of NDC codes... 67308-101-01, 67308-101-02, 67308-101-06
Source: https://ndclist.com/ndc/70621-101 and hhttps://ndclist.com/ndc/67308-101
Billing of these ALA HCl drugs to Medicare and commercial insurance companies involves billing both a combination of the J-code (to indicate the type of drug), how many units were utilized. Many of the carriers are now requiring inclusion of the manufacturer's NDC code for the particular packaging from the manufacturer. This is what creates the problem for generic compounded "Levulan"
Example for Levulan:
Physician performed PDT with 2 units of Levulan (Sun Pharmaceuticals). The following is billed with the NDC code for Levulan.
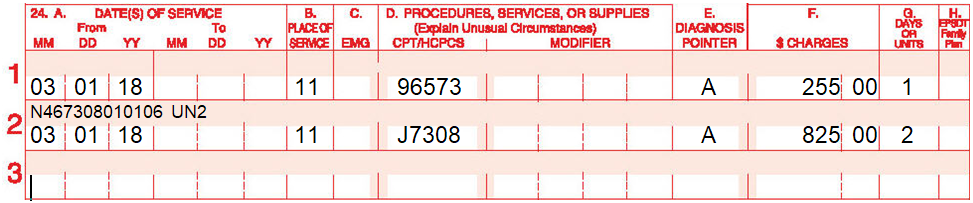
However, In the case of a compounded form of aminolevulinic acid HCl, several challenges exist.
1. In order for a generic to be billed using J7308, the compounding pharmacy would have to prepare and dispense the ALA in the same 354mg dosage as is described in the code's definition. Yes. this is "technically" possible, but would be a patent violation. Previously, DUSA (Levulan now owned by Sun Pharmaceuticals) challenged in the court system several compounding pharmacies that attempted this when the patent was still in place. Sun Pharmaceuticals is defending their patents on Levulan.
In addition, most carriers will require that the compounded drug included components (ingredients) of which the primary ingredient must also have been issued an NDC number. This usually occurs when the drug or ingredient is FDA approved. In other words, the generic ALA being used to compound must have an NDC number assigned to it. We are unable to locate an NDC number for generic ALA from any other manufacturer. Only the brand names for Levulan and Ameluz.
Because of the patent infringement issue, lack of NDC code for the generic ingredients, using J7308 is not possible for a generic.
2. Similarly, in order for a generic to be billed using J7345 (Ameluz), the compounding pharmacy would have to prepare and dispense the ALA HCl in the same 10mg gel form as is described in the code's definition. However, Biofrontera would likely challenge any compounding pharmaceutical company legally (much like DUSA did 8-10 years ago) as it would infringe on their patent for the drug. (Just like DUSA did in the past)
3. For a compounded form of the drug, however, the billing dermatologist could not utilize the existing NDC codes for Levulan or Ameluz. Those are assigned to the specific drug manufacturers and brand-name products, not generic.
How do you bill for compounded ALA HCL?
In reality, when billing for a compounded drug, a generic "unclassified drug" HCPCS code J3490 is submitted on the insurance claim, for which there is no set reimbursement. The billing dermatologist would need to also include direct cost information for the purchase of the medication from a compounding pharmacy.
Reimbursement is not guaranteed and could be denied completely. It is a risk that the dermatologist would take in trying this methodology.
Here is some guidance from Noridian Medicare (one of the Medicare contractors) on how to bill for compounded drugs.
Source: https://med.noridianmedicare.com/web/jeb/topics/drugs-biologicals-injections#2
In this next example, Blue Cross Blue Shield of Florida has specific guidelines that would prohibit the billing of a generic compounded form of ALA HCl. Here is what they say...
Compound drugs considered for reimbursement must meet all the following criteria:
• There must be a valid prescription order from a physician with at least one FDA approved
ingredient that has a recognized NDC number; AND
• There is no commercially available product comparable to the compound product; AND
• There is good evidence in the medical literature to support the use of all active ingredients; AND
• ALL active ingredients are prescribed for the specific diagnosis; AND
• The intended route of administration for the compounded prescription is supported by medical
and scientific evidence AND
• None of the active ingredients are addressed in another medical coverage guideline with
coverage limitations disallowing it to be considered a medical necessity
According to this, billing of a generic compounded form of Levulan would not be possible to BCBS of Florida because (no NDC code is assigned to generic ALA) and a commercially available product already exists!
Source: http://www.bcbsfl.com/DocumentLibrary/Providers/Content/PP_UnclassDrgs.pdf
Example of how to bill for unclassified drug:
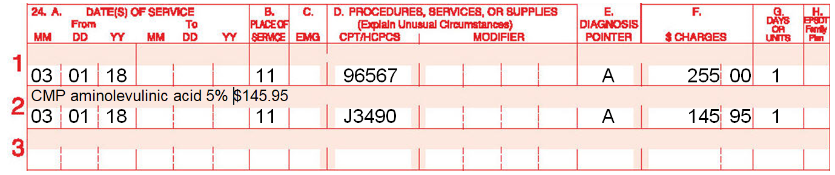
Furthermore, Medicare, and many commercial carriers will not allow the dermatologist to mark-up or increase the generic drug fee from the compounding pharmacy. It must be billed at the actual cost it was purchased from the compounding pharmacy. Therefore, other than lesser cost to the patient, there is no 'benefit' for a dermatology office to bill for the compounded form of the medication as there is no profit for the physician.
Lastly, the dermatologists increases malpractice risk and liability for use of a compounded drug/medication that is not FDA approved. What happens if the pharmacy mixes the compounded ALA HCl incorrectly? Who is liable for possible injury to the patient? The dermatologist or the pharmacy?
Tags:
Levulan, compound, j7308, compounding, CPT, legal, 96567, 96573, 96574, PDT, photodynamic, levulanic acid hydrochlorida
|